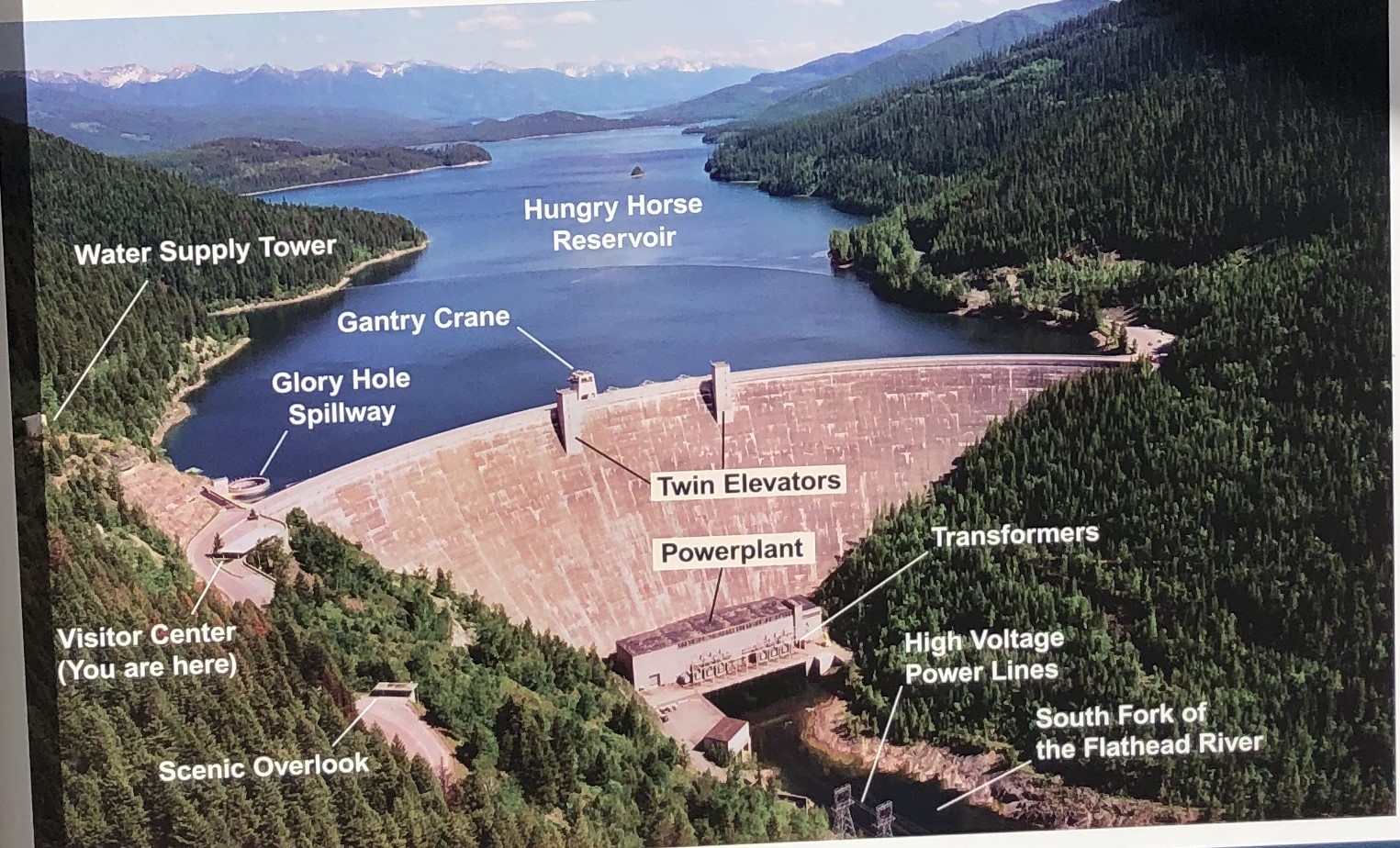
against the harsh rock peak, the delicate glass and wood of the lookout’s cabin stands defiant in the wind, a beacon in a sea of mountains
to lie down is to touch two of the four log walls
a dry bear grass bed on the wood slat floor
two chairs, one for the lookout and another for the ranger when he visits
instruments to measure fire direction and a man and the telephone line to bear witness
mules haul soda + yeast for the morning pancakes and letters to remember the world by
lard + milk and some ivory soap
a dozen candles to keep a flame to watch for fire by
streams seldom flow uphill; the smokechaser wakes at dawn break to fill his water bag miles below
upon the ascent, towards the top of the world, the world gets smaller
the alpine climate, above the tree line, the church spire tops of sub-alpine firs bow down to a forest in miniature
dwarf willows and mushrooms the size of fingernails and fairy spoons keep close to the ground in that windswept place
in the evening, the dispatchers open the telephone lines between the lookouts
they talk with the phones hung around their necks and call out checker moves, the hands free to play for two
* * *
and at night, the lightning
there is a smell to lightning, and a transmutative quality in its anticipation
coyote yips sound like human cries and then all is the crash of clouds
the wind washes you clean, sleeping in the slipstream
and that smell of static in the air, like mother drying clothes, of oxygen split to ozone
blind and searching, the blue energy seeks contact
lightning came in on the telephone line
the pathway of least resistance through the wire and then the water body
storm paths recorded faithfully by the earthbound
but with every added observation, reality looms larger and faith in prediction faulters
the recorder turned conductor and split asunder by his very own nature
the spirit may be displaced for some period, the forest of neurons lit momentarily by an alien fire
freeing of space again and maybe we return, and maybe the rolling clouds wash us clean
“lightning may do no damage to the wire, or it may burn the telephone wire completely”
mountain goats clamor back to the peaks
man sits at the telephone, about to speak
a dial tone, contact missed a beat
— Grace D.
* * *

This poem is inspired by recent events and old stories. While researching the Hungry Horse Dam for my last blog post, I came across two histories of the Flathead National Forest: The Flathead Story by Charlie Shaw and Trails of the Past by Kathryn McKay. Both histories are extensive and cover many aspects of early forest service happenings and 19th and 20th century life in the area. I was particularly drawn to the sections on fire lookout life and lightning. The quoted section in the poem, “lightning may do no damage to the wire, or it may burn the telephone wire completely” is quoted directly from Shaw’s chapter on lightning (The Flathead Story (Chapter 26)). The details in the poem concerning food rations and daily lookout life are also inspired by descriptions from both histories. I was moved by a tragedy that occurred near Pagoda Lookout involving two men maintaining a telephone line during a lightning storm. If you wish to read about this event, the story is in the last paragraph of Shaw’s chapter on lightning (The Flathead Story (Chapter 26)).

Last week I attended a local short film festival in Missoula, MT. One film that stuck with me was “The Lookout.” The film is not (yet?) available online, but if you are interested in details regarding the actors and the director, there is a profile on IMDb (The Lookout (Short 2024) – IMDb). Stay tuned for eventual public release of this film. The film opens on a lone lookout and his simple life. Strange, foreboding signs start to haunt him, bringing into question both his true nature and the environment’s true nature. People are not what they seem, and nature reclaims what was once believed separate. A still from the film of pancake batter dripping off a fire pit and clear secretions running from the lookout’s nose reveal an unknown, lurking presence. In the midst of all this creeping horror, there is great beauty in the landscapes and the natural world. I thought of this film, too, while writing this poem about the wonder and the terror of being outside, exposed to the elements.

The smell of lightening is very real, and it appears to be the smell of ozone (Cappucci 2018). For myself, lightning strikes a primal fear in me, and I have rushed down several trails to get away from a high alpine lake as dark clouds gathered around the peaks. On our drive back from delivering seed to the Coeur D’Alene Seed Nursery, my co-intern and I listened to a talk with Cathy Cripps, an alpine mycologist (Ep. 113: Mushrooms of the Rocky Mountains and Arctic Alpine Biome (fea – Mushroom Hour). She talked about her work in alpine climates and the world in miniature up there. She studied short willow forests and their mycorrhizal associations with alpine fungi and her pioneering work with mycorrhizal fungi in white bark pine restoration.

I discovered many things about the world and about myself this season. Thank you to the Flathead National Forest botany department, my co-intern Erynn, and many others who made this season so wonderful.

References
Cappucci, Matthey. “Lighting Has a Smell, and the Science Behind it is Beautiful.” The Washington Post. (2018). https://www.sciencealert.com/here-s-why-you-can-actually-smell-lightning
McKay, Kathryn L. “Trails of the Past: Historical Overview of the Flathead National Forest, Montana, 1800–1960.” Flathead National Forest. (1994). http://www.npshistory.com/publications/usfs/region/1/flathead/history/#:~:text=TRAILS%20OF%20THE%20PAST:%20Historical%20Overview
Shaw, Charlie. “The Flathead Story.” USDA Forest Service, Flathead National Forest. (1967) http://www.npshistory.com/publications/usfs/region/1/flathead/story/index.htm#:~:text=THE%20FLATHEAD%20STORY.%20By.%20Charlie%20Shaw.