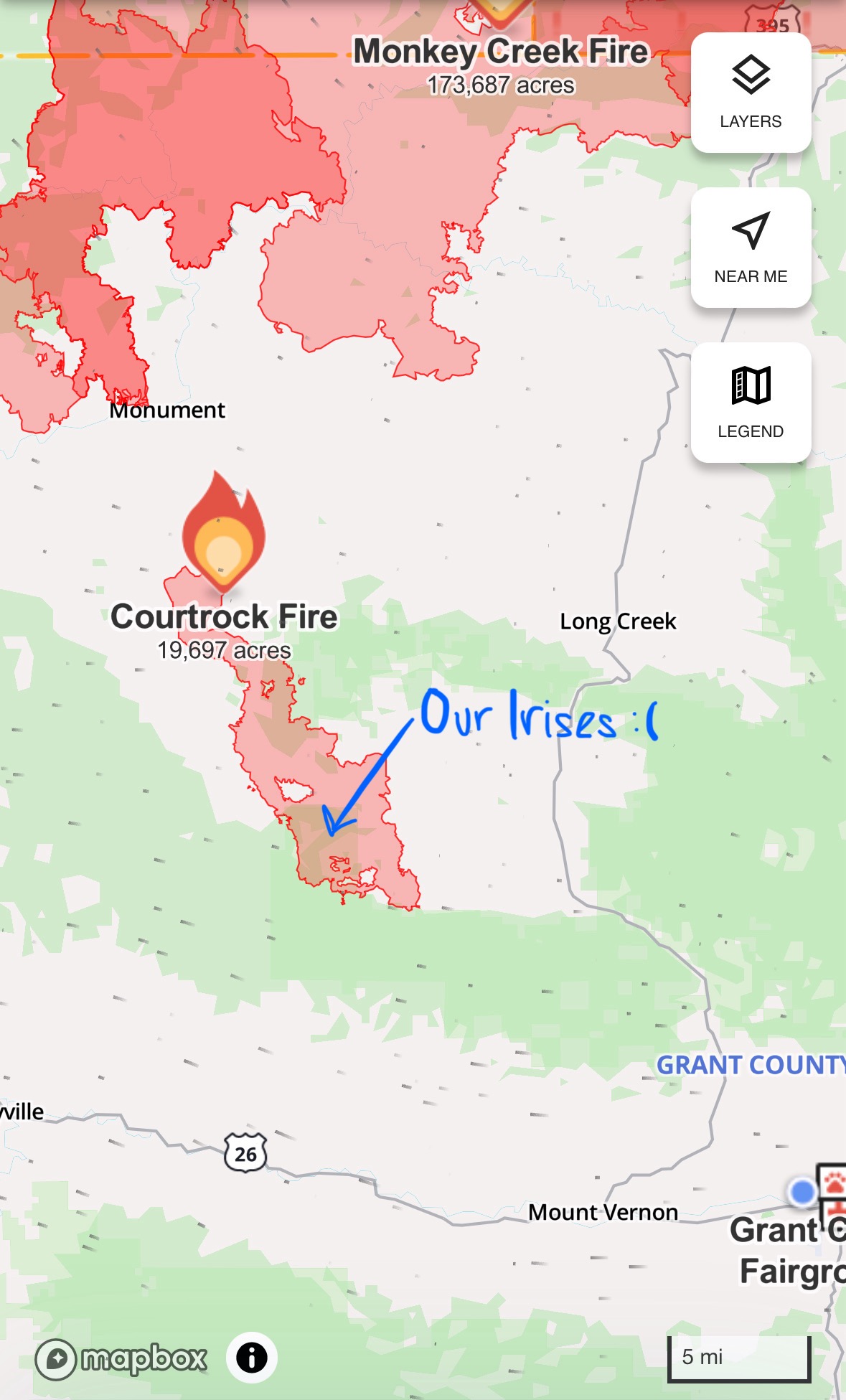
One of the most enjoyable activities so far at Midewin has been vegetation monitoring. We are collecting data to be used for floristic quality assessment, to monitor the quality and progress of our restorations. Each site that we monitor has several (4-5) 100 meter transects running north-south, each containing 25 points (spaced 4m apart). 1m*1m quadrats are placed at each point a random distance away from the transect (0-10m). At the start, a coin is flipped to determine whether the even random numbers will be east or west. Within the quadrat, every plant species is recorded, beginning with a .25m*.25m corner. After all species in the corner are recorded, all species present in the rest of the quadrat outside of the corner are recorded. Dead biomass (fuel) thickness is recorded at three points along the edge of the quadrat. Also, the species comprising 70%, 20%, and 10% of the dry weight in each quadrat are recorded.


Conducting vegetation monitoring requires plant identification knowledge beyond what I’d ever employed before in everyday botany. You must not only be able to identify mature plants in flower or in seed (which can be keyed out even if you have never seen the species before), but you must be able to identify every seedling in the understory, which is a daunting task. They will lack the floral parts often necessary to be keyable, and are best identified by someone who has grown the plants from seed, seeing them at every stage of their life. Luckily, I have grown many prairie species from seed at my native plant garden at Northwestern, taking special care to document the species in their seedling phase and putting the pictures in a native plant guide for the future generations of my Prairie Cats Ecological Restoration Club.

Still, many of the specimens I encountered in the field were completely enigmatic. The wetland plots were difficult, containing many wetland plants I had little to no experience with (all those silly little Lamiaceae). Grasses also presented a challenge — I had only ever paid attention to the floral characteristics of grasses (I have attempted to key numerous grasses from family, but the keys usually rely on floral characters). Foolishly, I had paid essentially no attention grass vegetative characters. However, thanks to Anna, our great botany technician, I was able to quickly learn how to distinguish some of the more common grasses. Also, the previous horticulturist at Midewin, Eric Ulaszek, is somewhat of a graminoid connoisseur and a great field botanist, and he created a vegetative key for the grasses of Midewin.

Every time I did quadrats, I derived a great amount of joy from finding the small, unobtrusive little things that you can expect to find in nearly every quadrat. For something like Poa pratensis (Kentucky bluegrass), finding it is almost a formality (it was in almost every single upland plot). However, they are small and often don’t show their inflorescences at the top, so finding them requires digging through all the small grass-like foliage at the bottom of the plot. I’d keep pulling little tufts of grass and looking for the Poas (also Poa compressa). And then all of a sudden… success! Poa on the Prairie! (This message is brought to you by the Don’t Kill Your Lawn Foundation).