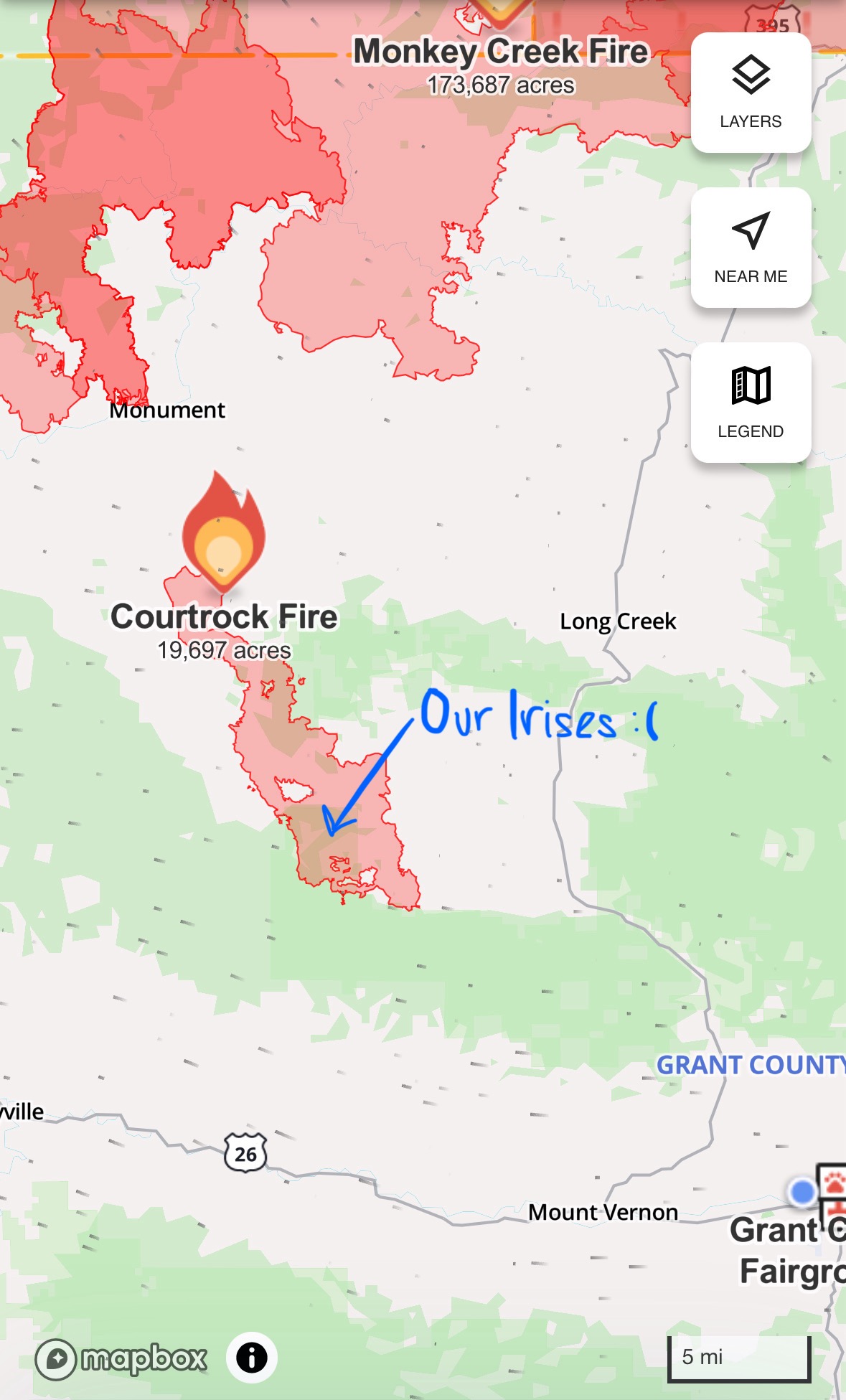
Fire season is early this year, and it has taken Oregon with a vengeance. Within one week of the first fire starting, every ranger district in Malheur had at least one large fire. South of our office, fire spans 170,000 acres and north of us, the fire is rapidly approaching 200,000 acres. In the two months that we’ve been here we’ve found only one population of Iris missouriensis, one of our key species, and it is now fully within the borders of a wildfire. For the past week, we watched the fire creep towards them and hoped, prayed, knocked on wood, and put all of our manifestation powers into the irises escaping unscathed. Unfortunately, that’s not how wildfires work. The smoke has also led to a couple lightning storms (which then led to more fires) with high velocity winds and some rain, though less than we would have hoped. All the elements are so prevalent to our daily lives here, it’s pretty cool.
Despite that blow, and the continuous blows of poor air quality, we have persevered. The fires have added an extra urgency to our collections and we are in go mode. With the help of the vegetation management team, we now have 22 seed collections which are rapidly growing. We’ve collected grasses and sedges and forbs, and paper bags of seeds have filled our perpetually insufficient storage space. The fires have cut into some other projects in the botany department too, so we’ve had double the amount of free hands to help. Silver linings! The growers that we will be sending our seed to need 500 grams of seed, which would definitely be difficult to achieve for some of these plants without so much help.
Now that we have so much seed, though, we’re starting cut tests, where we cut 100 seeds and count how many are germinable and how many are non-germinable to measure the viability of the population. We’re also counting how many seeds are in one gram of material and combining those two measures to figure out how much live seed we’ve collected. With the poor air quality, it’s been nice to have some work to do inside, but we’ve definitely been missing the full field days.


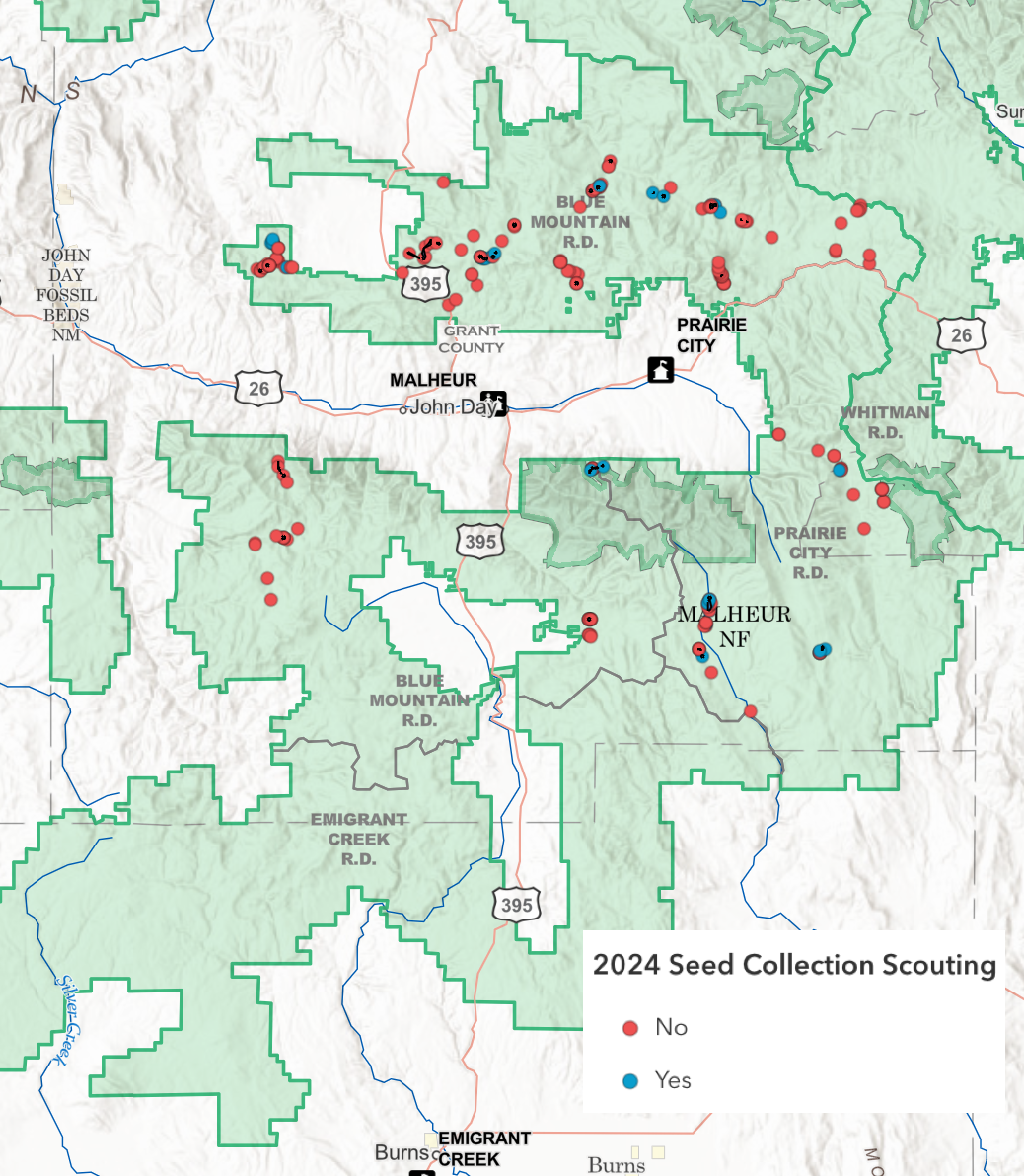
As of now we have over 200 scouting points on our map, 22 seed collections, and over 70 vouchers for potential collection spots.
Overall, we’re doing good and are excited to keep collecting.
Until next time,
Emma